By David Chandler, MIT June 24, 2014
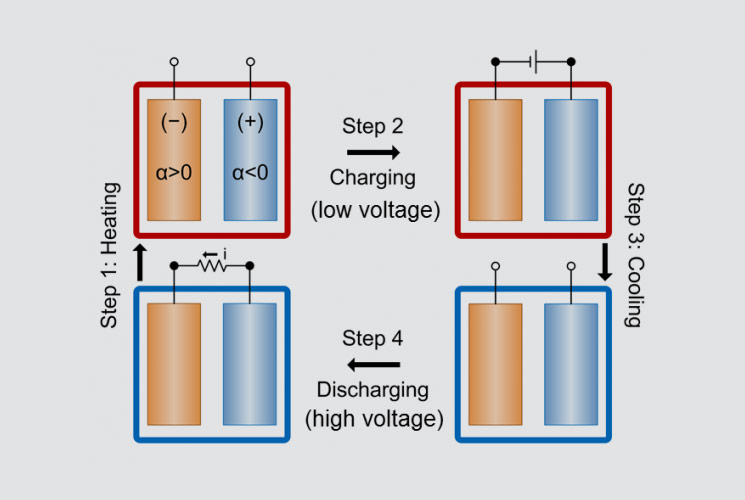
This diagram shows the stages of operation of a battery designed for heat harvesting. © David L. Chandler, MIY
A paper published in the journal Nature Communications, by postdoc Yuan Yang and professor Gang Chen at MIT, postdoc Seok Woo Lee and professor Yi Cui at Stanford, and three others, notes that the voltage of rechargeable batteries depends on temperature. Their new system combines the charging-discharging cycles of these batteries with heating and cooling, so that the discharge voltage is higher than charge voltage. The system can efficiently harness even relatively small temperature differences, up to about 100°C (212°F).
To begin, the uncharged battery is heated by the waste heat. Then, while at the higher temperature, the battery is charged; once fully charged, it is allowed to cool. Because the charging voltage is lower at high temperatures than at low temperatures, once it has cooled the battery can actually deliver more electricity than was used to charge it. That extra energy, of course, doesn’t just appear from nowhere: it comes from the heat that was added to the system. In a demonstration with waste heat of 60°C (140°F), the new system has an estimated efficiency of 5.7 percent.




